Thin data base
Biontech for children? This is what Stiko says about the possible emergency approval in the USA
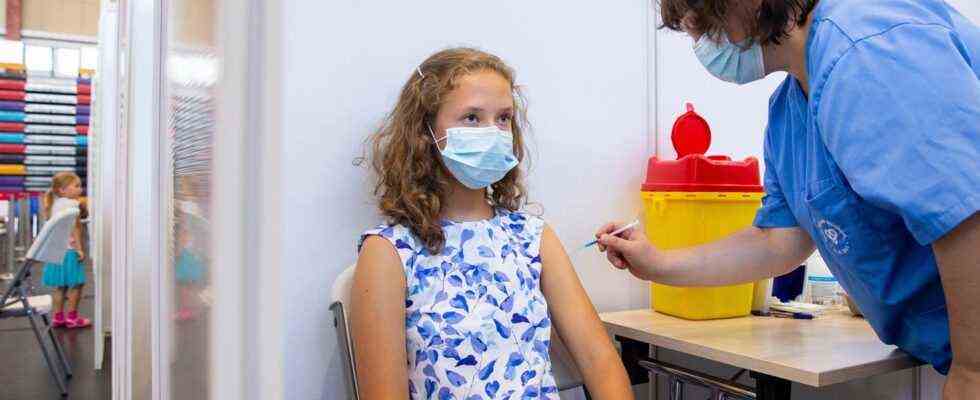
In Germany, the Stiko has not yet issued a recommendation for corona vaccination of children – but that does not prevent some parents from having their children immunized anyway.
© Raul Mee / AP / DPA
In America, experts are discussing an emergency approval of the Biontech / Pfizer vaccine for under 12s this week. In Germany, the vaccine could not be approved for children until mid-November at the earliest. Stiko experts mainly criticize the data basis.
An advisory panel to the US FDA is scheduled to discuss a possible emergency approval of Biontech / Pfizer’s corona vaccine in children this Tuesday. The recommendation for use in five to eleven year olds is not binding on the final decision, but the FDA usually follows the experts. The FDA’s final decision could be made within hours or days of the advisors’ recommendation. The CDC health authority then has to deal with it formally.
According to the White House, a vaccination campaign for the approximately 28 million affected children in the USA could start as early as November. The government will deliver 15 million doses of vaccine to paediatricians, clinics and pharmacies within a few days of approval, it said.
Biontech and Pfizer have also applied for approval of their corona vaccine for children of this age group in Europe, as they announced in mid-October. Accordingly, they transmitted the relevant data to the EU Medicines Agency (EMA).
The data basis is missing
According to Biontech / Pfizer, a clinical study showed that the vaccine was “well tolerated” for children of this age group and produced a “strong immune response” one month after the second dose. Compared to adults and adolescents, the five to eleven year olds were given a significantly lower dose of the vaccine. They received the second necessary spades after 21 days. 2268 children of the age group took part in the study.
For Germany, the question of evaluating the vaccine for children is still open, said the chairman of the Standing Vaccination Commission (Stiko), Thomas Mertens, of the German press agency. “We do not yet have a database for our assessment and recommendation.” First of all, the approval by the EMA is important, the time is still open. “Stiko has not yet seen and assessed the data from the registration study on safety and efficacy.” It is clear, however, that such a study with fewer than 3,000 test subjects could not record the risk of rare side effects.
The same problem arises for the panel as before the vaccination recommendation for 12 to 17 year olds, said Mertens. “Children have a very low disease burden from Sars-CoV-2. It is therefore important to weigh the expected positive effects and possible undesirable effects from the vaccination very carefully against each other.” Stiko will again carry out its own data analysis.
In the USA, more children at high risk of developing the disease
For Mertens, the starting position in the USA is not comparable to that here. “Children there are clearly more seriously ill with Covid-19. This may be due to the local health system and the higher proportion of children with risk factors such as metabolic syndrome or poorly controlled diabetes.”
The vaccination for 12 to 17-year-olds was originally recommended by Stiko primarily for children with certain risk factors for severe disease. These include, for example, being very overweight or chronic lung diseases. The general vaccination recommendation followed later, in mid-August. The federal and state health ministers had already agreed on broader vaccination offers for children from the age of twelve.
Could this be repeated with the younger children – first a vaccination offer from politicians and later the green light from Stiko? “We hope that doesn’t happen,” said Stiko member Martin Terhardt. “This is the only way to prevent renewed uncertainty and mistrust.” Vaccinations in this age group require careful and well-coordinated preparation with good data and reliable statements for parents as well as for vaccinating doctors.
Dramatic corona consequences for children
For the professional association of paediatricians, spokesman Jakob Maske does not expect EMA approval until mid-November at the earliest. “We will then wait for the Stiko recommendation.” As with the 12 to 17-year-olds, he initially counts on a recommendation for chronically ill children and possibly with an “optional rule” that also enables vaccinations for all other children. “The difficulty is that so far there is a lack of experience from other countries.”
When it comes to the question of the need for vaccination, according to the Berlin pediatrician, it should also be considered that the highest proportion of so-called PIMS cases is observed in five to eleven year olds: The pediatric multi-system inflammation syndrome rarely occurs overall as a result of the coronavirus. Infection, but requires hospital treatment.
Sars-CoV-2 is currently detected in Germany in particular in children and adolescents. The seven-day incidence in 5- to 14-year-olds was given by the Robert Koch Institute on Monday as 203, and the trend is rising. Long-term consequences are also feared by some.
Parental pressure increases
Mask reported that some of the parents were under great pressure to have their children vaccinated as soon as possible. There are doctors who specifically offer vaccinations in the so-called “off-label use”, ie even without approval for the age group. This is not illegal, but ultimately a question of security, said Maske. Overall, however, he assumes that the number of people vaccinated this way is very low.
It is to be hoped for the success of the vaccination campaign with children that politicians do not again put public pressure on the Stiko, said Maske. “This caused a lot of confusion among the 12 to 17 year olds and cost us paediatricians a lot of persuasion.”