A significant step forward for Valneva and hope for reducing the progression of the disease. The European Medicines Agency (EMA) has technically validated the marketing authorization application file for a vaccine against chikungunya, announces the Franco-Austrian manufacturer Valneva, whose headquarters is based in Saint-Herblain, near Nantes. This preventive vaccine, the first in the world, had already been approved in the United States on November 10. It was then presented as being intended for “people aged 18 and over at increased risk of being exposed to the virus”.
Valneva “welcomes” the technical acceptance of the EMA of its vaccine, currently called VLA 1553. This green light is a prerequisite for the possible availability of the vaccine to patients within 150 days, instead of 210 usually. A shortened deadline given “the major interest in public health and therapeutic innovation”.
“High risk of spread in Europe”
“The chikungunya virus has already spread to more than 110 countries and the risk of chikungunya spreading in Europe is relatively high due to the possibility of infected travelers. No vaccine or specific treatment is currently available for this debilitating disease, which therefore constitutes an unmet medical need,” reports Valneva management.
The vaccine on which the Nantes biotech is working is composed of a single dose. In the United States, it was marketed under the name Ixchiq. The global market for chikungunya vaccines is estimated to be worth more than $500 million per year by 2032.
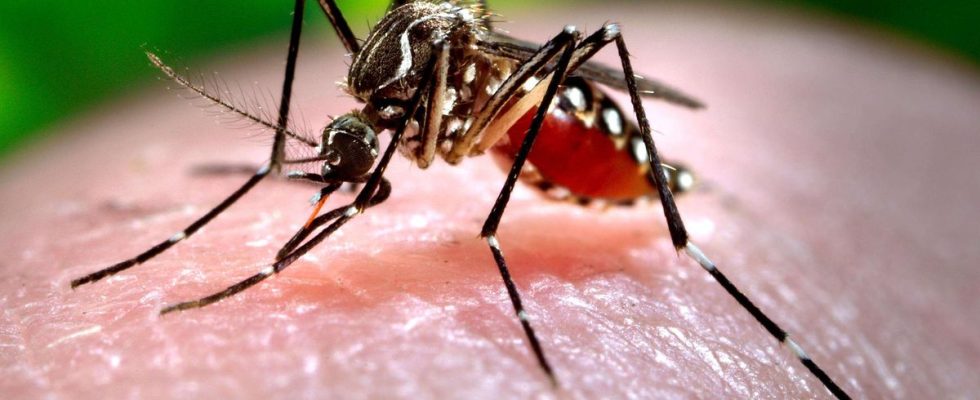
Appearing for the first time in Africa in 1952, chikungunya is a virus transmitted from human to human via mosquitoes of the genus Aedes, of which the famous tiger mosquitoes. The disease manifests itself after an incubation of four to seven days on average. A high fever (above 38.5°C) appears suddenly, accompanied by headaches, aches or joint pain, which can be intense, mainly affecting the extremities of the limbs, describes the Ministry of Health. There is no specific curative treatment for the virus.