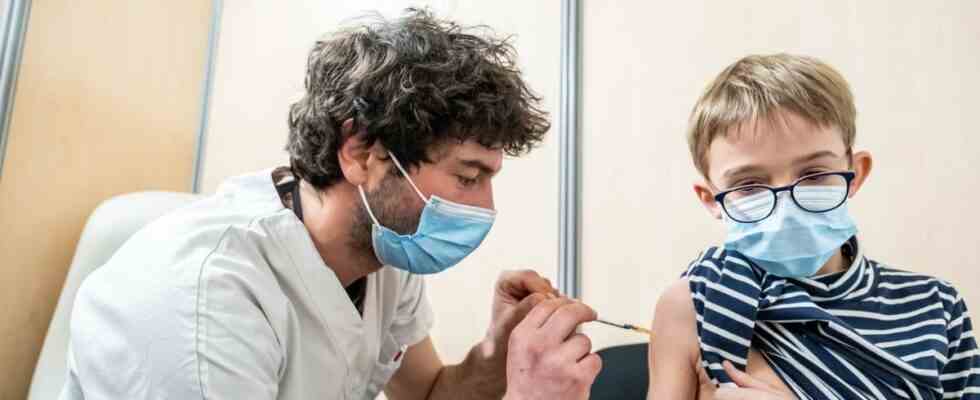
Vaccinated 5-11 year olds may soon have a booster. The Pfizer-BioNTech alliance is preparing to file an application for authorization “in the coming days” in the United States, then elsewhere in the world for its anti-Covid vaccine for children.
The trials conducted by Pfizer-BioNTech notably analyzed the blood of children who received this booster dose approximately six months after the second. After the injection, the levels of neutralizing antibodies against the Omicron variant, currently dominant in the world, increased 36 times compared to the levels observed after the second dose.
Clinical trials for children under 5 still awaited
The two initial doses for children aged 5 to 11 have been authorized by the United States Medicines Agency (FDA) since the end of October. The dosage used for this age group is 10 micrograms, both for the initial injections and for the booster dose (compared to 30 micrograms for ages 12 and older).
Pfizer and BioNTech “plan to submit an application for emergency use authorization for a booster in children ages 5-11 in the United States in the coming days,” according to a joint statement. “The companies also plan to share this data with the European Medicines Agency (EMA) and other regulatory agencies around the world as soon as possible,” the statement added.
For children under 5, the results of clinical trials of this vaccine are still awaited, after the companies announced that they wanted to test an initial series of three doses for them. Indeed, for these very young children, a dosage of only 3 micrograms per injection was chosen, but the immune response triggered after only two doses was then not sufficient.