(K)A question of genes: As is well known, tumors arise from mutations or damage to our DNA, which cause the cell to degenerate. But purely epigenetic changes in the genome can also apparently trigger cancer, as a research team reports in “Nature”. These chemical deposits on the genome lead to tumors by blocking or enhancing the reading of genetic information. The genes themselves remain unchanged, but their regulatory mechanism is disrupted.
Cancer is one of the most common causes of death worldwide. What the countless different types of cancer have in common is that certain cell types get out of control and grow unchecked. The trigger is usually damage to the chromosomes or DNA mutations that impair the function of proteins and other cellular processes. Cancer is essentially a genetic disease – at least that’s what people have thought for the last 30 years.
But in addition to DNA, the epigenome also plays an important role in cell function and possibly cancer, as recent studies suggest. These chemical deposits on the DNA strand regulate which genes are read in a cell’s genome and thereby determine which properties and functions this cell receives – for example, whether it functions as a neuron or skin cell. But how big is the influence of epigenetics on tumor formation and can cancer arise entirely without permanent DNA mutations?
Gene switch in sight
A research team led by Victoria Parreno from the University of Montpellier in France has now investigated these questions using fruit flies (Drosophila). To do this, the doctors intentionally caused epigenetic changes to the animals’ genome and then remedied them. They concentrated on the so-called Polycomb proteins. These epigenetic switches regulate various genes and signaling pathways that are important for cells. In many types of human cancer, there is a defect in these switches.
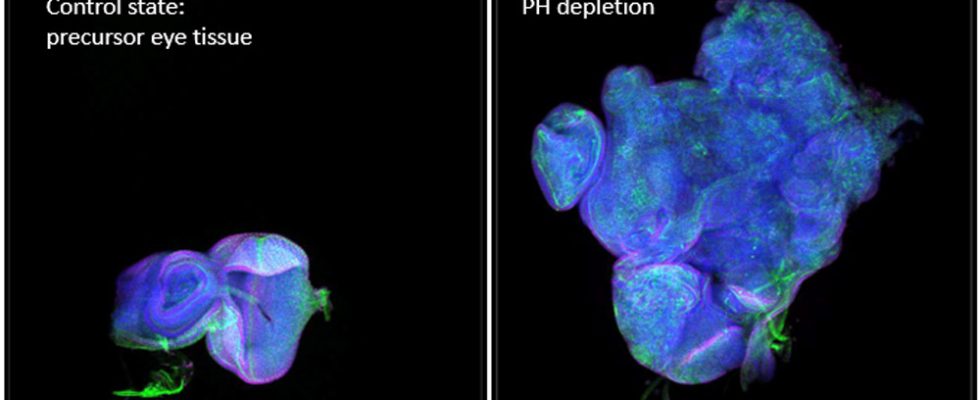
Parreno and her colleagues blocked these Polycomb proteins in the developing eyes of fly larvae using a molecular inhibitor. After 24 hours, they removed the inhibitor and released the Polycomb proteins again. Meanwhile, the researchers observed the genetic and cellular effects this temporary intervention had.
Long-term active genes lead to cancer
The tests revealed that after this back and forth, part of the fly genome no longer responded to the Polycomb proteins and instead self-regulated its expression. A few dozen genes became independent of their epigenetic switch and were permanently more or less active than normal, as the team reports. As a result, among other things, more signaling proteins from the group of cytokines were produced and an important cellular signaling pathway (JAK-STAT), which leads to cell division, was constantly activated – the cell degenerated.
This caused a tumor to form in the fly tissue, which continued to grow even after the epigenetic signal that originally triggered it was reversed. The gene sequences themselves remained unchanged, as controls showed. The larvae died eleven days after tumor growth began. In adult flies that were implanted with such a tumor, however, the cancer spread further and led to aggressive metastases, as Parreno and her team report.
Paradigm shift in oncology
The study shows for the first time that cancer can also be caused exclusively by epigenetic factors. These lead to a “self-sustaining epigenetic state that supports tumor growth,” writes biologist Anne-Kathrin Classen from the University of Freiburg in a comment on the study. Gene mutations are therefore not absolutely necessary for tumor development. The findings thus justify a paradigm shift in cancer medicine.
At the same time, the findings could open up new possibilities for personalized cancer therapy. To do this, however, it must first be examined whether the findings also apply to humans. “Whether these findings also apply to more complex organisms such as mammals remains to be seen,” says Classen. “In humans, temporary epigenetic changes can arise from environmental influences that are specific to a person’s life history, such as certain diets or medications or exposure to chemical substances.” But whether these changes actually lead to cancer in us remains unclear. (Nature, 2024; doi: 10.1038/s41586-019-0000-0)
Source: University of Montpellier/CNRS
April 26, 2024 – Claudia Krapp