“Soldier” bacteria filled with poison sacrifice themselves for the benefit of their fellow animals, giving them disease-causing properties
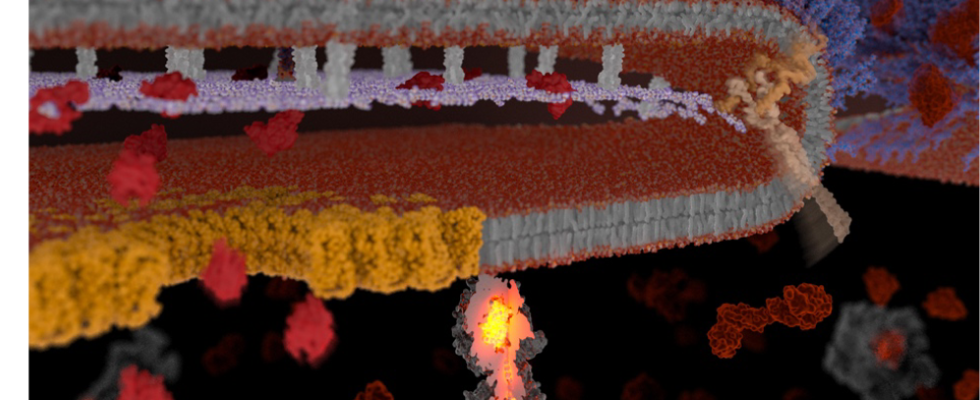
Disease-causing bacteria release toxins when they infect a host. Before these deadly poisons can attack the host cells, the bacteria must first export them from their place of production – the cell interior – via special secretion systems. The group led by Stefan Raunser, director at the Max Planck Institute for Molecular Physiology in Dortmund, has now elucidated a previously enigmatic and unusual secretion mechanism that enables the release of so-called Tc toxins. In a sort of kamikaze attack, a small group of “soldier” bacteria, filled to the brim with toxins, release their deadly payload by exploding inside the host.
© MPI MOPH Secretion mechanism of Tc toxins. © MPI MOPH
Once a pathogenic bacterium has invaded its host, it initiates a series of defense and attack mechanisms. This includes the secretion of a number of toxic proteins that undermine the host’s cellular defenses. This allows it to spread, penetrate deeper tissues and organs and colonize them.
In gram-negative bacteria, which can cause severe infections and are increasingly developing resistance to antibiotics, the toxic proteins must overcome multiple cellular barriers – both those of the bacteria and those of the host – to ultimately reach their target. For this purpose, bacteria have developed a number of specialized secretion systems. While some of these can secrete a variety of toxins and are found in almost all bacteria, other systems have only been identified in a few bacteria. The mechanism for the secretion of many smaller toxins is already known.
Not so with larger ones, like the Tc toxins from the notorious ones Yersinia-Bacteria are produced, which also include the pathogens of plague and tuberculosis. “For decades it was a mystery how the giant Tc toxins got to their destination. In our previous work, we elucidated the first 3D structure of a Tc toxin using cryo-electron microscopy. We have already been able to find out how the toxin breaks through the last barrier, the host membrane, using a syringe-like injection mechanism. Now we have been able to complete the picture and show how these toxins overcome the three barriers that separate the interior of the bacterium from its surroundings in a truly spectacular way,” says Stefan Raunser.
Exploding bacteria
© MPI MOPH
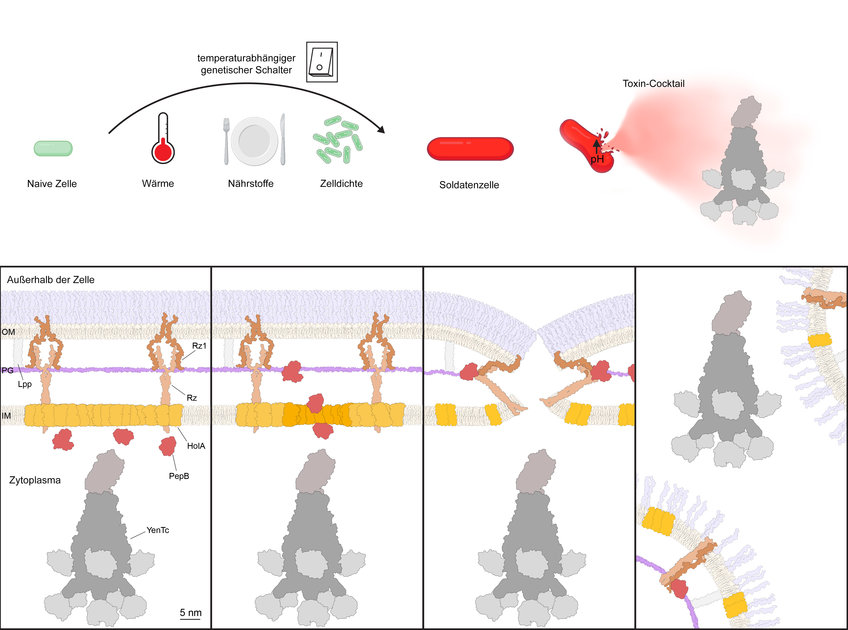
Model for soldier cell differentiation and YenTc release. Top image: Naïve cells transform into soldier cells by activating a temperature-sensitive genetic switch in response to the insect host’s nutrients, temperature, and increased cell density. A change in pH leads to activation of the secretion system and release of the toxin cocktail. Bottom picture: The steps of YenTc release in detail. © MPI MOPH
In their recent work, Raunser and his team combined several cutting-edge techniques to study the secretion of the Tc toxin YenTc. This is caused by the pathogen Yersinia entomophaga produced that infects insects and plays a significant role in the manifestation of the infection. The biggest challenge for the researchers was initially to find out which of the known secretion mechanisms the bacterium uses. For this purpose, the researchers switched off all possible secretion systems one after the other using targeted genome editing.
When none of the knockouts stopped the release of the toxin, the toxin was modified using the same technique so that its secretion could be visualized – this time successfully. “Seeing some of the bacteria literally explode and release their toxins was a real Eureka moment,” says Oleg Sitsel, lead author of the study. Careful proteome analyzes finally identified a recently discovered pH-sensitive type 10 secretion system that is responsible for the release of the toxin. Cryo-electron tomography was then used to examine the details in detail show how this secretion system exports cellular contents step by step via a previously unknown lytic mechanism of action – and thus overcomes the three barriers that surround gram-negative bacteria.
The transformation into a soldier cell
The researchers found that only a small, specialized subset of bacterial cells produce and export the toxins and pay for it with death. But what causes these cells, which the authors refer to as “soldier cells,” to first enlarge and produce a deadly toxin cocktail containing YenTc, and then commit suicide for the benefit of their comrades? The researchers initially determined that the appearance of soldier cells is dependent on temperature, nutrients and cell density.
They then discovered a temperature-dependent genetic switch that synchronizes the production of the toxins with the production of the secretion system, turning “normal” cells into soldiers. Mass production of toxins and cell enlargement ensure that only a few individuals need to be sacrificed for the good of the bacterial population – an extremely efficient strategy.
“We suspect that normal cells transform into soldier cells during feeding in response to the insect host’s nutrients. Toxin secretion is pH-sensitive, which delays release until the soldier cells reach the alkaline posterior midgut, their place of deployment,” explains Raunser. “This secretion strategy is unique. The behavior of these bacteria shows features such as differentiation and altruism that are reminiscent of eusocial systems. If this turns out to be a common mechanism, we may have uncovered a weak point in the bacteria: targeting the soldier cells could be a promising solution medical strategy in the fight against pathogenic bacteria, especially in times of increasing resistance to antibiotics,” says Raunser.