Off for the bearer of hope
The Tübingen company Curevac buries its first corona vaccine – how it could get that far
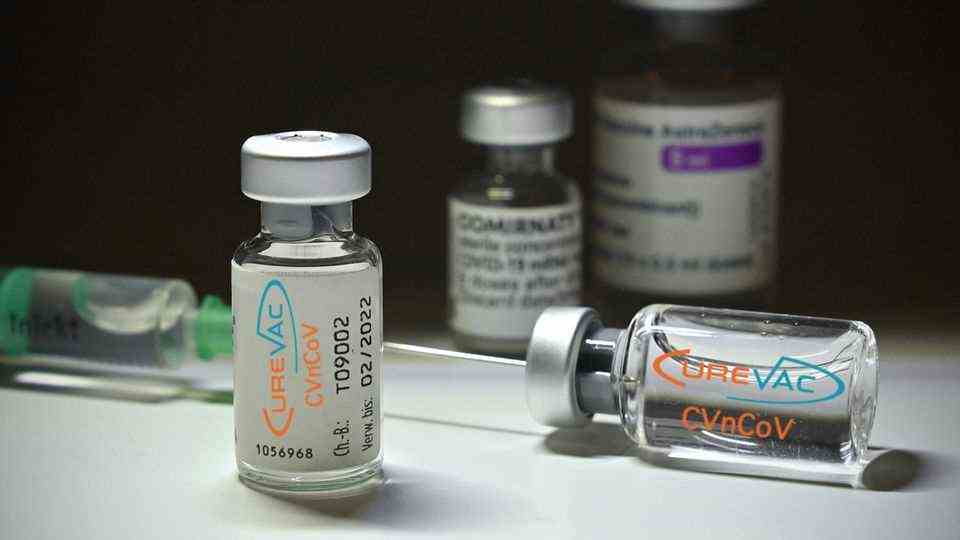
The first generation of a corona vaccine from the Curevac company has failed – now the work continues.
© Frank Hoermann / SVEN SIMON / Picture Alliance
The Tübingen biotech company Curevac has drawn a line. His first-generation corona vaccine candidate will not come on the market, but will land in the bin. How could it come to this?
When the manufacturers’ race for a corona vaccine began, Curevac was right at the forefront. The Tübingen company was considered a pioneer in mRNA technology, so the hopes that were placed on the German vaccine candidate were correspondingly high. Hopes that must now be buried for the time being. The biotech company is withdrawing its first corona vaccine from the approval process. Instead of in the arm, the vaccine now ends up in the bin. For Curevac it is the end of a race with many stumbling blocks – but by no means the end. The company still wants to have an ace up its sleeve. A second candidate is in the works.
Failure was looming. At the end of June it became known that the CVnCoV vaccine would not be able to compete with the vaccines that have already been approved. The effectiveness left a lot to be desired. At that time, however, it was still assumed in Tübingen that the European Medicines Agency (EMA) would still grant approval. That is now off the table. On Tuesday, the EMA announced that it had terminated the process of reviewing continuously submitted data following notification from Curevac. A final analysis had shown that the vaccine was only 48 percent effective against Covid 19 disease.
Dependent on the competition
How did it happen that the pioneer was left behind? In March 2020, even before the first lockdown, rumors were already circulating that the then US President Donald Trump wanted to bind the Tübingen biotech company exclusively to the USA. Excitement spread.
During this time, Curevac founder Ingmar Hoerr traveled to Berlin and spoke to Health Minister Jens Spahn about the new mRNA technology. The next day, Hoerr collapsed in his hotel room – an aneurysm. It was March 13, 2020, a Friday. Hoerr was in a coma for six and a half weeks. It took months before he was fit again. Months in which other vaccine developers overtook Curevac by the right. Hoerr’s misfortune came at an inopportune time, but it is only one of many, many reasons that contributed to the failure of the corona vaccine project.
Infectiologist Peter Kremsner headed the global Curevac vaccination study, in which around 40,000 people took part. In an interview with the “Wirtschaftswoche” he reported a series of problems. Accordingly, the scientists had difficulty holding the test subjects. Because while research was still being carried out in Tübingen, other vaccines were already approved. “Of course, we gave our test subjects the option of being vaccinated with the vaccines that were now available,” he reported. The result: a huge dropout rate. 32 percent of the participants between 18 and 60 years of age ended the study prematurely, and 70 percent of the older participants.
Lots and lots of problems
And then there was the problem with the app. The participants should document possible vaccine side effects in an app. But that was exactly what caused trouble over and over again. “We have often received feedback from test persons that it is difficult to enter the undesired effects into the app. But we have tried to help as far as possible,” said Kremsner. “How many reports of side effects have been omitted as a result, I am unable to say, hopefully few.”
But these are only minor details compared to what brought the vaccine candidates to the end. Because it just didn’t work the way you’d hoped it would. “It was the low dosage,” says Kremsner. Accordingly, the vaccine was dosed significantly lower than the competing products. Due to its nature, however, it was not possible to turn it.
Curevac is already making new plans
Curevac’s path in corona vaccine development has been a bumpy one so far. That should change in the future. Because despite this defeat, nobody thinks of giving up, on the contrary. Curevac now wants to focus on the development of further, better Covid-19 vaccines with its British partner GlaxoSmithKline (GSK). Among other things, these should offer longer-lasting protection against new variants in a single vaccination. The CEO Franz-Werner Haas announced on Tuesday.
“We see that with CVnCoV we can no longer be part of the first wave of pandemic vaccines. But we have a firm intention to lead the way with our partner in the development of a new, improved second-generation vaccine,” said Haas . The new goal: the official approval of an improved Covid-19 vaccine in 2022. Compared to the first vaccine, CV2CoV – the name of the new development – shows up to ten times higher immunogenicity in animal models.