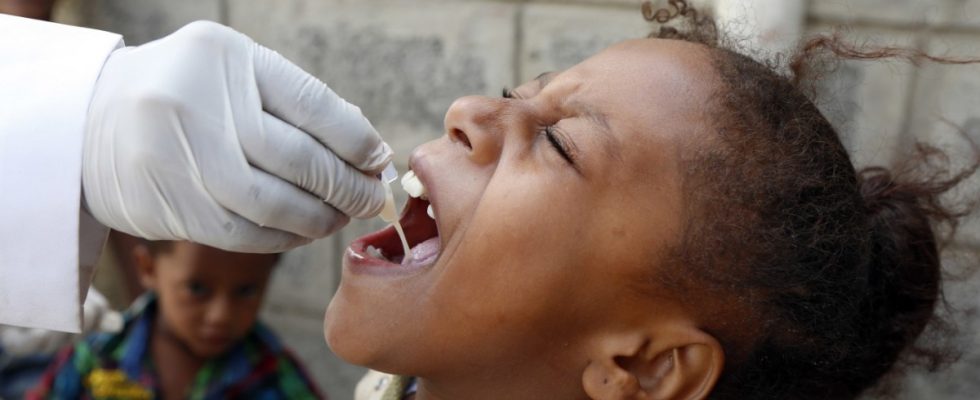
The number of cholera cases worldwide has risen so rapidly that there is a risk of a severe shortage of vaccines. As the ICG coordination group reported on Wednesday, last year countries applied for twice as many vaccine doses as were produced. The ICG monitors and distributes global vaccine supplies. Measures are urgently needed to prevent further outbreaks and ramp up vaccine production. According to the World Health Organization (WHO), the South Korean company Eu-Biologics is the only one currently producing a vaccine against cholera.
Last year, 36 million doses were produced, but at least 72 million were requested by countries, the WHO reported. More vaccine doses were requested between 2021 and 2023 than in the entire previous decade combined. The WHO is part of the coordination group, as are the UN children’s fund Unicef, the aid organization Doctors Without Borders and the Federation of Red Cross and Red Crescent Societies.
Cholera cases have been increasing since 2021. In 2022 there were more than twice as many cases as in the previous year, a total of 473,000, according to the WHO. Preliminary data for 2023 suggested there were more than 700,000 cases. Acute intestinal infection is transmitted through food and water contaminated with feces containing the Vibrio cholerae bacterium.
Outbreaks occur when hygiene conditions are poor. This often happens after natural disasters or in conflict regions when many people are driven from their homes. The most severely affected are the Democratic Republic of Congo, Ethiopia, Haiti, Somalia, Sudan, Syria, Zambia and Zimbabwe.
In view of the high demand for vaccines, the coordination group had already issued the recommendation in October 2022 to use one vaccination dose instead of the usual two doses. This protects more people, but doesn’t last as long. The group is therefore pushing for more investment in wastewater systems and the supply of clean drinking water. On the other hand, new vaccines must be approved as quickly as possible and brought onto the market in sufficient quantities and at affordable prices.